FDA revised its food allergen labeling guidance.

On 7th January 2025, the US Food and Drug Administration (FDA) revised for the fifth time its food allergen labeling guidance.
As a guidance, this document is not amending the Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) but is updating the FDA thinking on its interpretation. However the FDA view is changing substantially: therefore, despite the non-binding nature of guidance documents, it is better for the food industry to be updated about such changes.
Let’s see the more impactful Q&A.
- FDA does not intend to move forward with the thresholds for the declaration of food allergens or to ease the use of PAL (precautionary allergen labeling, e.g. may contain, not regulated under FALCPA)
“A.4 Do the food allergen labeling requirements of the FD&C Act require FDA to set so called “thresholds” for any food allergen?
No, the food allergen labeling requirements of the FD&C Act do not require FDA to establish a threshold level for any food allergen. FDA has previously examined the topic of thresholds but has not established specific thresholds for any food allergens. See https://www.fda.gov/food/food-labeling-nutrition/approaches-establish-thresholds-major-foodallergens-and-gluten-food.”
2. FDA does “consider “milk” as milk from domesticated cows, goats, sheep, or other ruminants”. Also, any ingredient that could contain milk protein (e.g. lactose), shall be subject to FALCPA labeling requirements. On top of that:
“milk and milk ingredients from animals other than cows should also include the name of the animal source, such as “goat milk” and “whey (goat milk)” in the ingredient list or “Contains goat milk” in a separate “Contains” statement, or both.”
3. The “tree nuts” list has been considerably shortened! Some quite annoying nuts — from an allergen management perspective — are missing: namely chestnut, shea nut, and COCONUT!
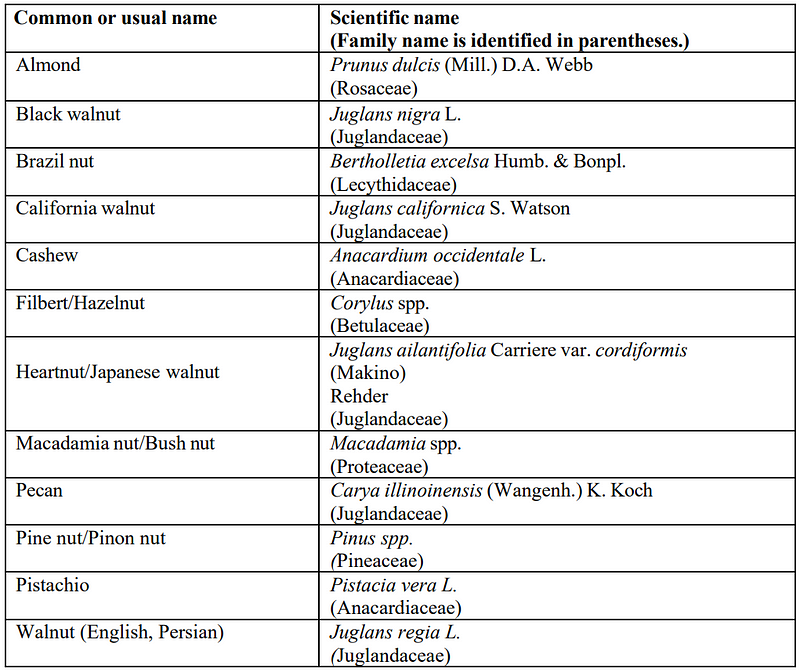
This is certainly easing harmonization between the lists of other major countries like the EU and Canada: for more see the post of my colleague and co-founder in Food Orbit, Dr. Bert Popping!
Only the tree nuts listed in the table are considered major food allergens, and, therefore, must meet the food allergen labeling requirements.
Because other tree nuts that are not listed in the table do not have a robust body of evidence to support inclusion as a major food allergen, they should not be included in the “Contains” statement, even if they are used as ingredients because the “Contains” statement is reserved for major food allergens. Tree nuts used as ingredients, but not listed in the table, would still be required to be listed by common or usual name in the ingredient list (21 CFR 101.4).
4. An interesting point that is worth a reminder is finally how to label multiunit packages.
D.3 Under the food allergen labeling requirements of the FD&C Act, must individual units within a multiunit package have a “Contains” statement if each unit is not fully labeled?
The unit containers in a multiunit or multicomponent retail food package are exempt from certain labeling requirements, including an ingredient statement under 21 CFR 1.24(a)(14); however, such foods are not exempt from allergen labeling requirements. If the food is or contains a major food allergen, a “Contains” statement must be used in order to comply with section 403(w)(1) of the FD&C Act and should be declared near the statement of identity in the absence of an ingredient list. However, no labeling is needed, including allergen labeling, if the individual unit is an unlabeled inner sleeve intended solely for protection of the product, such as sleeves of crackers, and does not contain any written, printed, or graphic matter.
That’s all, for now!
Discover more from FOOD LAW LATEST
Subscribe to get the latest posts sent to your email.
